Austin, May 01, 2025 (GLOBE NEWSWIRE) — Mycoplasma Testing Market Size & Growth Analysis:
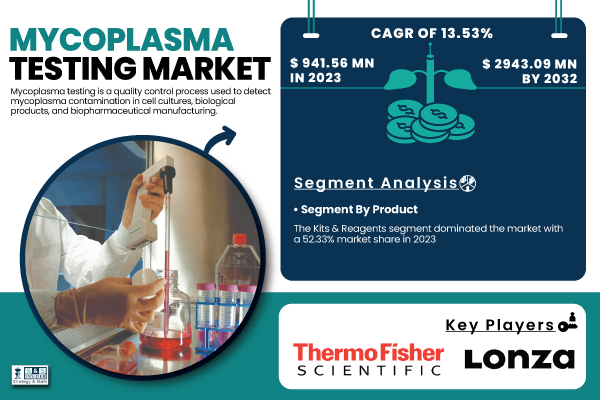
According to SNS Insider, The Mycoplasma Testing Market was valued at USD 941.56 million in 2023, is projected to reach USD 2,943.09 million by 2032, expanding at a compound annual growth rate (CAGR) of 13.53% between 2024 and 2032. This growth is being driven by heightened concerns around contamination in biologics and cell culture-based products, alongside increasing adoption of cell-based therapies and vaccines, which demand highly sensitive and accurate mycoplasma detection methods.
Get a Sample Report of Mycoplasma Testing Market@ https://www.snsinsider.com/sample-request/5761
In the United States, the market demonstrated solid momentum, accounting for USD 322.13 million in revenue in 2023. It is expected to grow at a 13.37% CAGR over the forecast period. This strong performance is supported by stringent FDA regulations on microbiological testing, rising investment in next-generation biologics, and the expanding role of contract manufacturing organizations (CMOs). With a robust pharmaceutical pipeline and proactive measures to minimize contamination risks and avoid costly product recalls, the U.S. continues to be a key driver of global market growth.
A main driver of the mycoplasma testing market is the growing use of biologics in therapeutic development, especially in oncology and immunology. These treatments demand aseptic manufacturing settings, so contamination detection is both operationally and regulatory necessary. Mycoplasma testing is a required quality control step in all human biological products according to the U.S. FDA’s Biologics Guidance (2023), which has driven demand for dependable, quick, and cost-effective testing tools.
Key Mycoplasma Testing Companies Profiled in the Report
- Thermo Fisher Scientific Inc. (MycoSEQ Mycoplasma Detection Kit, MycoAlert Mycoplasma Detection Kit)
- Lonza Group Ltd. (MycoAlert PLUS Mycoplasma Detection Kit, Nucleofector Technology)
- Merck KGaA (PlasmoTest Mycoplasma Detection Kit, Mycoplasma Removal Agent)
- Charles River Laboratories International, Inc. (Endosafe-PTS System, Accugenix Mycoplasma Testing Services)
- Roche Diagnostics (MycoTOOL Mycoplasma Real-Time PCR Kit, LightCycler 480 System)
- bioMérieux SA (VIDAS Mycoplasma Assay, NucliSENS EasyQ Mycoplasma)
- Agilent Technologies, Inc. (AriaMx Real-Time PCR System, Mycoplasma Detection Assays)
- Becton, Dickinson and Company (BD MAX Mycoplasma Detection Kit, BD Phoenix M50)
- InvivoGen (PlasmoTest Mycoplasma Detection Kit, Normocin Antimicrobial Reagent)
- PromoCell GmbH (Mycoplasma Detection Kit, Antibiotic-Antimycotic Solution)
- Sartorius AG (Microsart ATMP Mycoplasma Kit, Microsart RESEARCH Mycoplasma Kit)
- Takara Bio Inc. (PCR Mycoplasma Detection Set, Mycoplasma Elimination Cocktail)
- Minerva Biolabs GmbH (VenorGeM Mycoplasma Detection Kit, Mycoplasma Off Kit)
- ATCC (American Type Culture Collection) (Universal Mycoplasma Detection Kit, Genuine Cultures for Mycoplasma Testing)
- Biological Industries Israel Beit Haemek Ltd. (Mycoplasma Detection Kit, Mycoplasma Prevention Reagent)
- MP Biomedicals, LLC (Mycoplasma PCR Detection Kit, Mycoplasma Removal Agent)
- Clongen Laboratories, LLC (Mycoplasma Testing Services, PCR Detection Kits)
- GenBio (ImmunoWELL Mycoplasma IgG/IgM Test, Mycoplasma Pneumoniae IgM ELISA)
- ScienCell Research Laboratories, Inc. (Mycoplasma Detection Kit, Mycoplasma Removal Reagent)
- Eurofins Scientific (Mycoplasma Testing Services, Mycoplasma Real-Time PCR Assay)
Mycoplasma Testing Market Report Scope
| Report Attributes | Details |
| Market Size in 2023 | US$ 941.56 million |
| Market Size by 2032 | US$ 2943.09 million |
| CAGR | CAGR of 13.53% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Key Regional Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East]), Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia Rest of Latin America) |
Segment Insights
By Product
The Kits & Reagents segment is dominant in 2023 with a 52.33% share in the global market. For the quick, dependable, and simple solutions these products provide for identifying Mycoplasma contamination in cell cultures and biological samples, the growing inclination for commercially accessible ready-to-use kits has drastically cut the time and expenses related to regular testing.
Because of strict regulatory standards and the increased focus on contamination control in GMP-compliant facilities, biopharmaceutical producers depend more and more on reagent kits for both internal and outside testing. Furthermore, driving the expansion of the segment is the great need for research reagents in clinical and academic research establishments. Expected to keep their supremacy over the forecast period are technological breakthroughs, including lyophilized reagents, multiplexing kits, and PCR-enhanced kits.
By Technology
With a 34.12% share in 2023, the PCR (Polymerase Chain Reaction) segment dominated the market because of its great sensitivity, specificity, and short turnaround time. The gold standard in many QC processes, since PCR-based detection enables quick identification of Mycoplasma DNA even at low quantities.
Large-scale pharmaceutical manufacture and clinical research labs, as well as other high-throughput testing environments, find the method perfect for automation and real-time monitoring. Further predicted to enhance the adoption rate by enhancing quantitative detection capabilities and lowering false positives are innovations in digital PCR (dPCR) and multiplex PCR. PCR-based technologies are expected to remain fundamental in quality assurance in the pharmaceutical and biotechnology sectors as regulatory authorities suggest molecular detection more and more.
By End Use
With a 36.12% share in 2023, Pharmaceutical & Biotechnology Companies dominated the market. These companies rely on aseptic manufacturing settings and rigorous adherence to regulatory norms, so they are the main users of mycoplex testing. The increasing pipeline of biologics and biosimilars supports the segment’s growth and calls for strict quality control at every manufacturing level.
These firms are substantially investing in upstream and downstream contamination testing and integrating automated mycoplasma detection systems in their manufacturing pipelines as global acceptance of cell and gene treatments rises. Moreover, the move toward individualized medicine and the application of new biologics such as monoclonal antibodies and CAR-T treatments has increased the demand for strong, quick, validated microbial testing approaches.
For A Detailed Briefing Session with Our Team of Analysts, Connect with Us Now@ https://www.snsinsider.com/request-analyst/5761
Mycoplasma Testing Market Segmentation
By Product
- Instruments
- Kits & Reagents
- PCR Assays
- Nucleic Acid Detection Kits
- Stains
- Elimination Kits
- Standards & Controls
- Others
- Services
By Technology
- PCR
- ELISA
- Direct Assay
- Indirect Assay
- Microbial Culture Techniques
- Enzymatic Methods
By Application
- Cell Line Testing
- Virus Testing
- End of Production Cells Testing
- Others
By End-use
- Academic Research Institutes
- Cell Banks
- Contract Research Organizations
- Pharmaceutical & Biotechnology Companies
- Others
Regional Analysis
North America held a 40.25% share in the global mycoplasma testing market in 2023, driven by major biopharma companies, high healthcare expenditure, and a strong regulatory framework. Regional supremacy has been strengthened by the U.S. FDA’s demand for mycoplasma screening prior to product introduction and significant funding in cell therapy manufacturing facilities. Moreover, research and development money from companies like the NIH and BARDA has inspired creativity in microbial testing techniques.
Expanding biopharmaceutical R&D in nations such as China, India, South Korea, and Japan would help the Asia Pacific region to have the quickest CAGR over the projection period. Government programs such as India’s Biotechnology Industry Research Assistance Council (BIRAC) and China’s 14th Five-Year Plan are giving financial support and incentives to improve biologics manufacturing capability. These advances have raised regional demand for technologies for pollution detection and control.
Buy a Single-User PDF of Mycoplasma Testing Market Analysis & Outlook Report 2024-2032@ https://www.snsinsider.com/checkout/5761
Table of Contents – Major Key Points
1. Introduction
2. Executive Summary
3. Research Methodology
4. Market Dynamics Impact Analysis
5. Statistical Insights and Trends Reporting
5.1 Contamination Incidence and Prevalence (2023)
5.2 Mycoplasma Testing Trends (2023), by Region
5.3 Testing Volume, by Region (2020-2032)
5.4 Healthcare & Biopharma Spending on Mycoplasma Testing, by Region (2023)
6. Competitive Landscape
7. Mycoplasma Testing Market by Product
8. Mycoplasma Testing Market by Technology
9. Mycoplasma Testing Market by Application
10. Mycoplasma Testing Market by End-use
11. Regional Analysis
12. Company Profiles
13. Use Cases and Best Practices
14. Conclusion
Related Reports:
Pharmaceutical Sterility Testing Market
Biopharmaceuticals Market Forecast
Clinical Diagnostics Market Report
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company’s aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.









